Sticky Situations
Researchers have discovered a Candida auris-specific adhesin that mediates skin and inserted medical device colonization
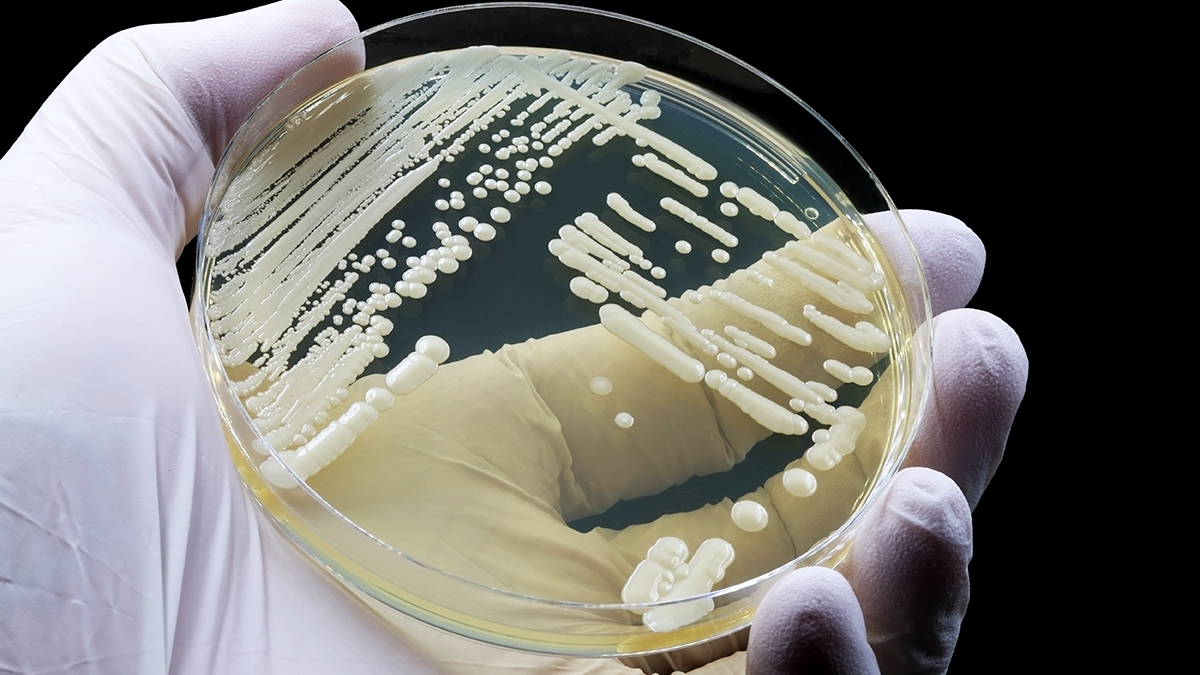
Cases of Candida auris have been on the rise since 2016 and, by 2022, it had secured its place in the “Critical Priority Group” of the World Health Organization’s first ever list of fungal priority pathogens. It is typically found in healthcare settings and spreads through contact with infected patients or contaminated surfaces, including medical devices, bedside tables, bed rails, and more. With a crude mortality rate of around 30 percent, researchers and healthcare organizations are desperately trying to find out all they can about the fungus.
Before colonizing surfaces or skin, fungal pathogens must first stick to them, which is largely the responsibility of fungal adhesins. In a recent study, researchers have investigated the role of C. auris adhesins in colonization (1) – and we spoke with senior author Teresa O’Meara to find out more…
What inspired your study?
As a field, we are only beginning to understand where C. auris might exist in nature, as opposed to in the clinics. Something has likely led to simultaneous adaptations that have allowed C. auris to start infecting humans and causing outbreaks; as we learn more about C. auris’ biology and get more information about its natural reservoirs, we should be able to make stronger inferences about its enigmatic emergence.
One of the major, shocking observations in the early days of C. auris outbreaks was that the fungus seemed to colonize everything in healthcare settings once it was introduced. Surveillance would find it on bed rails, in ceiling tiles, on clothing – and all of this seems to create a reservoir to fuel outbreaks. We wanted to know how C. auris associates with surfaces on a molecular level, and we were interested to find that it encodes its own specialized adhesin – surface colonization factor 1 (Scf1) – for this purpose.
How does Scf1 differ from other known fungal adhesins?
Scf1 is unusual because it uses cation-pi interactions to bind to surfaces. Most fungal adhesins use hydrophobicity (and, in our study, we found that the adhesin IFF4109 uses this mechanism); however, it looks like SCF1 behaves more like attachment proteins from mussels, barnacles and other marine organisms. Given that only C. auris and Candida haemulonii have Scf1 proteins in their genome, there are many open questions about where this gene came from.
The adhesins we characterized in this study seem to be critical drivers of medically relevant traits – colonization of catheters, model surfaces, and skin, as well as being important for C. auris disease progression. In light of this, we were very surprised to find that different strains of C. auris express SCF1 to very different levels. In some strains, it is among the strongest gene expressed, while in others it is hardly turned on at all. We are still investigating why this might be, but the potential clinical consequences are intriguing.
What are the next steps for your research?
We are interested in understanding why C. auris would adapt to express Scf1 or not, and when that adaptation is happening. We have also only solved one piece of the puzzle here by investigating surface colonization; in reality, outbreaks are very complex and are a moving battle between colonization, persistence, and transmission for C. auris. A question that remains unanswered is about how Scf1 contributes to virulence – we see a strong impact, but the mechanisms are still being investigated. In the long term, hopefully we can fill in some more knowledge gaps about the peculiar behavior of this organism and start to help the medical community make meaningful adjustments to therapeutic approaches.