I Test the Pains Down in Africa
How rats are helping to migrate a neglected tropical disease across West Africa – and how the labs are battling to diagnose it
Click here to read Part I of this article.
On the rural roads of Kenema, Sierra Leone, a lone rider blazes a trail with a special parcel in tow. It weighs less than a handful of pennies, but it’s worth ten times its weight in gold. The motorcyclist has a long way to travel – possibly 200 miles – but getting there could mean life or death. That’s because someone is waiting for a Lassa fever test, and speeding along the dusty tracks is the only way to get it to them on time.
Lassa fever is a zoonotic disease endemic to West Africa. Although often symptomless, the disease has a 15 percent fatality rate in those who are hospitalized due to infection. Vague or non-existent symptoms make diagnosis practically impossible without laboratory testing – not something that the poor rural communities of West Africa have much access to.
The problem is exacerbated by climate change. The virus is spread through human–rat interaction, and increasing extreme weather events are leading rodents to make haste into populated areas (1), boosting the chances of infection and potential death. What’s more, the disease is spreading outside of its traditional endemic locale.
But all is not lost. Though the issue has been growing, so have efforts to quash it. The WHO, the CDC, and a plethora of other groups have been working to bring testing to the region, including Zalgen Labs, which played a fundamental role in creating a reliable test. The company now has its sights set on a vaccine.
We spoke with Luis Branco, Managing Director and co-founder of Zalgen on how it launched laboratories in Sierra Leone and how exactly its two-wheeled diagnostic delivery service came into existence.
How did you get involved in Lassa fever testing?
The story dates back to 2004, when I was collaborating with some colleagues who worked on high-containment pathogens. I was introduced to Robert Gary out of Tulane University, who had a long-standing presence in the world of virology. Robert was interested in exploring opportunities in the viral hemorrhagic fevers space – where Lassa and others like Ebola belong. The aim was to identify pathogens that were largely neglected but could have a very high potential for a natural pandemic. He wanted people on the team who had different skills. I was something of a gene jockey at the time, putting genes together, cutting and pasting them and making proteins out of them – all that kind of good stuff. So I was brought onto that team and we got going with the help of some NIH funding.
We aimed to develop new-age recombinant-based diagnostics for these diseases. Very little had been done throughout the years and there really weren’t any good diagnostics. The ones that existed required extensive safety testing and needed high containment BSL-4 labs, so they couldn’t be commercialized. Instead, our idea was to develop some solid, rapid, bedside tests that we could use in the field.
We wanted to give medical facilities in Lassa-endemic countries the chance to save lives through diagnosis. So, we started looking at other aspects of Lassa fever to aid our understanding of the disease itself, including the ecology, the interaction between rodents and humans, human and rodent genetics, how people respond to infection, and so on.
One of the funding sources from the NIH was keen to understand how people develop Lassa antibodies. Out of that emerged the largest-known collection of human-derived antibodies against Lassa fever. We went to individuals who had been lucky enough to survive the disease and asked them for a sample of their blood so we could mine it for antibodies that had potential to develop into a therapeutic. I should note that my whole career prior to becoming part of this program was antibody based – I was involved in a number of programs that were aimed at driving antibodies against viral agents. With Lassa, we looked at our collection of antibodies and thought, “We may have a therapeutic here.” We ran some preliminary studies that showed that these antibodies were protective in animal models of the disease, which opened the door for funding opportunities. So, Gary and I decided to found Zalgen. We have stayed very active in the space of diagnostics while continuing to develop therapeutic drugs for Lassa.
When did you start working in situ in Africa?
We put boots on the ground from day one. We developed diagnostics by doing work stateside in our laboratories here and then we took trips – sometimes several a year – to areas like Sierra Leone and Nigeria, where we helped develop laboratory infrastructure that would permit us to actually evaluate these diagnostics on-site. Obviously, this was where the disease and the patients are located, so we needed access to relevant samples in situ.
CEPI is the Coalition for Epidemic Preparedness Innovations, which was created to create vaccines for pathogens with some of the highest pandemic potential – one of which was Lassa.
Once the tests had a certain level of extensive validation, we started distributing them to other entities that worked on Lassa fever; CEPI and the WHO came along to give legs to these initiatives. Throughout the years, people had worked on Lassa vaccines, but none had proceeded to licensure. Yet people had put out platforms, so CEPI funded a number of initiatives on a competitive basis, and we got an award from CEPI to provide all the diagnostics that were necessary to conduct those studies.
The diagnostics are important to understand the prevalence of Lassa in the region and to measure the potential immune response to a vaccine. These diagnostics identify active Lassa infections, but also determine whether or not a person had been previously infected with the virus. CEPI also funded a study called the Enable Lassa research program – the largest surveillance program to date (2), which, among other things, aims to answer a key question: how far and wide is Lassa fever present in that region? Again, we supply the diagnostics for that. The program has multiple sites throughout West Africa that collect a lot of samples – they bring them in, they test them, they derive the data, and then put a picture together.
Once completed, it gives us a sense of how widespread the disease is, while setting the scene for vaccine studies. Obviously, you need to have naive populations – people that have never been exposed – so you can see what kind of immunity they develop from vaccine exposure and not from viral exposure. And that’s why this study is still ongoing, so we can understand where the hot spots are and where they have the best population centers to choose for future clinical trials.
What was it like going to do field work?
I have a lot of stamps on my passport! And those are the moments that really define a career, right? It was not just about going in and having access to samples and to a patient population, we put in a lot of work. We’re talking about hammer and nail type of approaches, where infrastructure was built; brick and mortar facilities with sustainable resources, like water and electricity and proper cooling so that we could have laboratories with equipment that needed certain temperatures to operate. There was also lots of community building. We needed drivers who could take us around the country and personnel who could bridge the gap between cultures and social norms. We needed to understand the interaction between the rodents and the humans, so we had ecology teams and support personnel.
How and where did you start setting up facilities?
Our first location of interest was Kenema in eastern Sierra Leone. It’s one of the hotspots for Lassa fever. The WHO and the CDC did have a presence there for a long time until the civil war broke out in the early 1990s and they had to leave the country for safety reasons. That set the stage for us returning to Kenema down the line.
Many of those first few trips in early 2005 were on unpaved roads. It would take about 10 to 12 hours to reach Kenema – today it takes about three and a half. The infrastructure was lacking and the facilities really were very poor. For a long time, we operated on tiny amounts of small-town-supplied power for water and electricity – it was very sporadic. We ran some operations by just burning fuel; we had these old UN generators to power the facilities and part of the hospital. There was a lot of adapting to circumstances.
Later on, we installed large arrays of solar panels that provided a good amount of energy all day long while recharging batteries, which allowed us to continue work the next day. We also built a brand new Lassa ward – a wing of the hospital that was specifically designed to accept infected patients on one side, have them treated, and allow them to recover on the other side. Things have improved dramatically since then. Huge investment in Sierra Leone means that there’s regular access to power and fiber optics for high speed internet, for example, The “good old days” of us trying to figure out how to supply power for an experiment are gone.
When it comes to Lassa fever, we’ve learned more in the last 10 years than in the previous 50 combined. These viral and hemorrhagic fevers, especially Lassa and Ebola, have seen a tremendous boost not just in knowledge, but also in countermeasures.
Why is Lassa considered difficult to diagnose?
It was difficult because diagnostics were not available. Everybody learned about PCR during the pandemic, right? You could go to your local testing center, have a swab up your nose and then run a test. But that kind of technology requires complex instruments and the people with the knowledge to run them properly. For example, you need a lab that can maintain room temperature, which was just not possible or sustainable in places like Sierra Leone.
And that’s why we developed and continued to sustain the use of rapid tests – the kind that people can get from a pharmacy and run themselves. There might be better infrastructure in places like Kenema and other places throughout West Africa, but there are still huge distances for people to travel. Imagine if you were in London but you had to go to Liverpool to get tested. Imagine making your way there in difficult conditions without a car or public transport.
Our solution is networks of medical personnel that know Kenema well. These days, a medical officer can place a call to Kenema and say, “I have a patient here we suspect has Lassa.” We have a team member who gets on a motorbike with a little packet and rides out to them. If it is Lassa fever, they’ll call for an ambulance to bring them to Kenema to be treated on the Lassa ward. It’s an incredibly valid and appropriate way to diagnose. Once at the ward, we can confirm the Lassa fever diagnosis in the lab with other tests like PCR.
Just as we all know how powerful it was for the general population to be able to go to a pharmacy and get the SARS-CoV-2 rapid tests, it’s equally powerful for the community living with Lassa to get diagnoses this way.
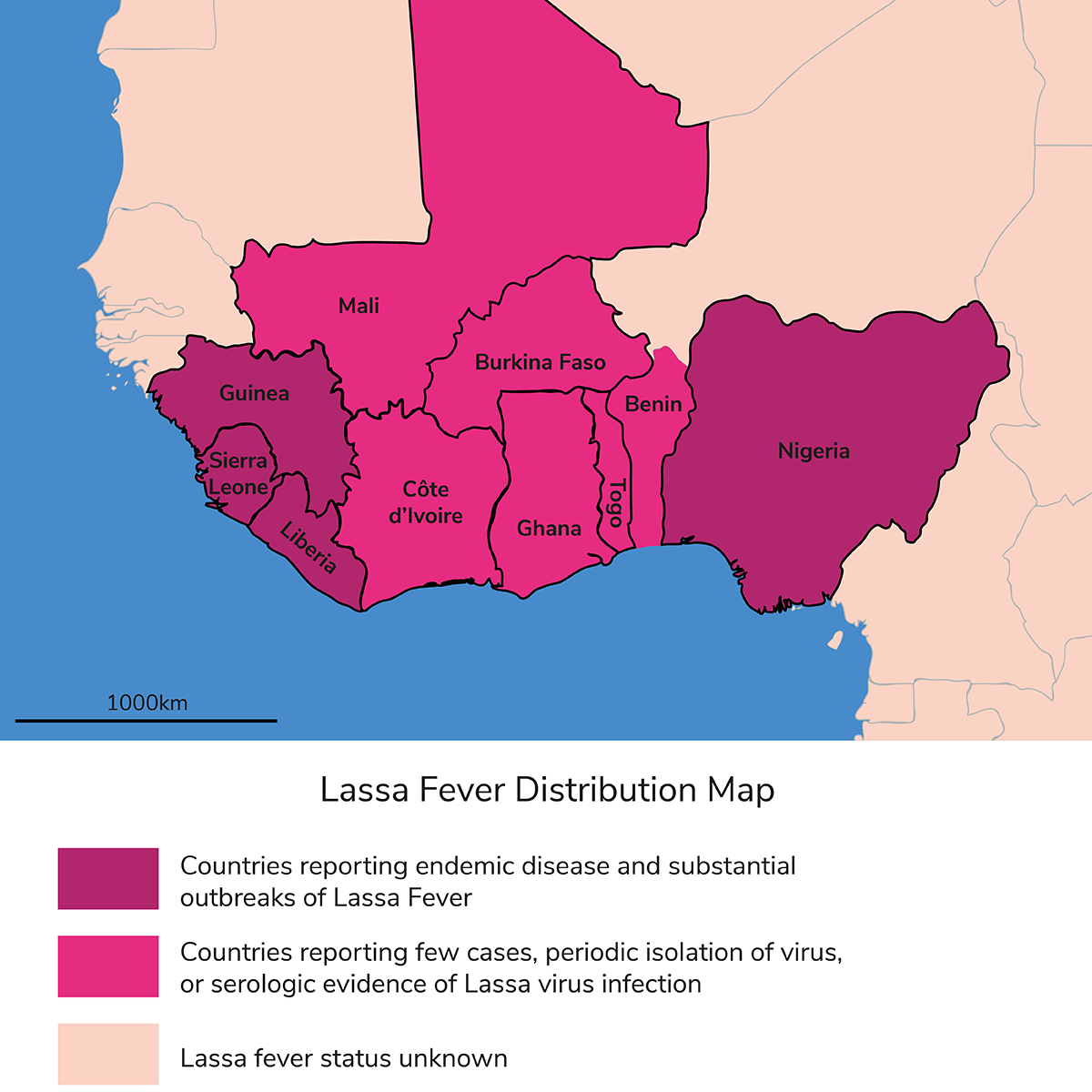
It’s no longer difficult to diagnose Lassa, but there are still many obstacles in distributing diagnostics. The countries in which Lassa is endemic – Sierra Leone, Liberia, Guinea, Burkina Faso, Ghana, and even all the way out to Nigeria – are some of the lowest GDP countries in the world. They really don’t have the resources to procure and then sell these types of diagnostics in-house. One aspect that we always highlight is the fact that none of these countries have ever bought a single diagnostic from us. And to be clear, when I say we commercialize these tests, we sell them to vaccine developers, NGOs, and organizations who can provide financial support. Everything that lands in these countries is free for the people that need and use them.
How is the changing climate affecting the spread and distribution of Lassa?
I know that not everybody believes in climate change and its impact, but, if I take a completely neutral stance, we cannot negate the fact that the rodent reservoir is expanding. We know that even from 15 years ago when we started going to Sierra Leone and Nigeria; we have seen a dramatic expansion in exposure to the virus and the numbers of people getting sick. There are many more reported cases, and rodents and humans are certainly coming into contact a lot more. When we started over a decade ago, we knew that Lassa fever was prevalent in Sierra Leone, Guinea, and Liberia. On the other side of that belt, in places like Nigeria, there was evidence for zero prevalence. You could go there and test some people who might have antibodies against the virus, possibly because of someone who traveled through. Today, we’ve got significant expansion of Lassa fever; there are people succumbing to it throughout all of Nigeria and now Togo and Benin. In fact, there are new strains that have emerged from Benin and Togo, so we know there has been expansion.
How do you adapt tests for these new strains?
A fantastic question! We’re constantly chasing that problem. When we started this whole program, our focus was to simply get it up and running. To that end, we focused on the prevalent Sierra Leone lineage of the virus, which is called lineage four. We developed all of our platforms around lineage four, but when we brought our tests to Nigeria the tests didn’t perform very well. And that’s because two different lineages are more prevalent in Nigeria: lineages two and three.
We realized that we needed to develop what we call a pan platform – something that was going to be applicable to just about every lineage of Lassa that we knew about or could imagine. We’re now at the point where we have largely discontinued our first-generation product. It works well in Sierra Leone, but we’re not taking any chances.
When the Togo and Benin strains emerged, we immediately jumped on them. It’s a living, breathing platform.
We scientists are sometimes labeled as fanatics because we have these “crazy” ideas about great pandemics that could wipe out the world. But every now and then, nature shows us that these things do happen.
It’s up to us in worldwide public health to make sure we have the tools to tackle the problem. It’s the worst thing in the world to say we could have solutions, but we didn’t work on it when we had the chance; we didn’t pay attention to it and now we are months or years away from a countermeasure. Just look at COVID-19 to see how many people succumbed to the disease simply because we weren’t prepared. We’re trying to build countermeasures for Lassa now – before it’s too late.
References
- S Cadmus et al., “Ecological correlates and predictors of Lassa fever incidence in Ondo State, Nigeria, 2017–2021: an emerging urban trend,” Sci Rep, 13, 20855 (2023). PMID: 38012226.
- S Penfold S et al., “Enable Protocol authorship group. A prospective, multi-site, cohort study to estimate incidence of infection and disease due to Lassa fever virus in West African countries (the Enable Lassa research programme)-Study protocol,” PLoS One, 30, 18, (2023). PMID: 36996258.